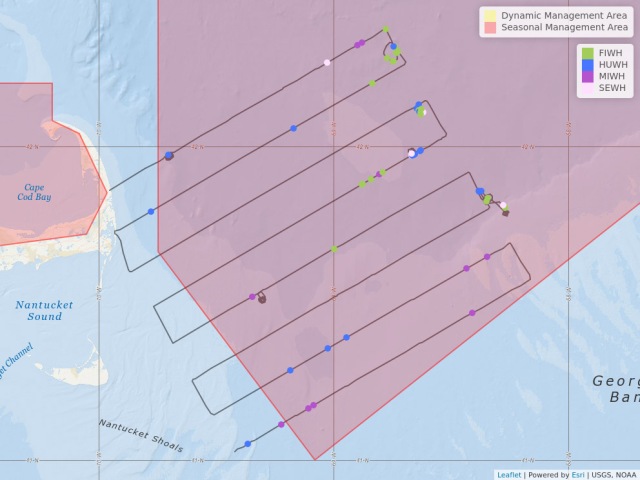
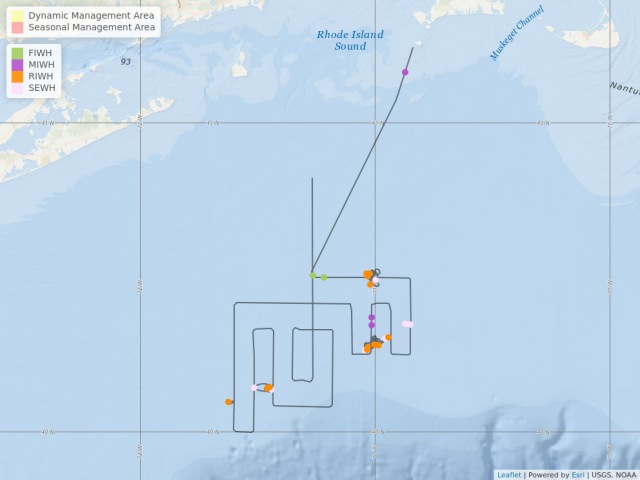
The 2019 spring right whale cruise is being conducted aboard the University of Connecticut’s research vessel Connecticut, which is a 90-foot vessel proving to be a very nimble and capable vessel for this work. We departed Woods Hole on Monday, May 6, around 6pm, arriving in an area south of Martha’s Vineyard and even south of the New York shipping lanes by daylight Tuesday morning. The weather was good and the NEFSC aerial crew came out in the NOAA Twin Otter to survey in the same area. They relayed positions of two groups of right whales, each a group of two. We traveled to the first group and found one right whale in that area. Conditions were not good for launching the small rigid-hulled inflatable boat, or RHIB, but with the help of Pete Duley’s 500mm lens, we managed identifiable images from the fly bridge of the ship. We then headed west to the second location, where we again found one right whale.

Pete Duley with his 500mm lens. Photo credit: NOAA Fisheries/Elizabeth Josephson
The next morning, Wednesday the 8th, we were set up a few miles to the west of the aerial team’s last sighting from the 7th. Conditions were calm and we set up a boxed survey around an area where five right whales had been seen. As we worked our way east of the original sighting, we came upon several small groups of sei whales. They were all skim feeding. Obtaining sei whale biopsy samples is a secondary objective of this cruise. We decided to launch the RHIB and get some biopsy samples. As fieldwork often goes, by the time we launched, the sei whales were no longer skim feeding and the seas began to pick up.
After some effort to keep up with sub surface feeding sei whales (we don’t recommend that you try this at home!) we brought the RHIB back onboard…..er, just in time for Genevieve Davis to call from the fly bridge with a right whale sighting! Whaaaat?!? Again, we were able to photograph from the fly bridge.
Unfortunately, we couldn’t relaunch again in these seas. We photographed three right whales from the ship this day. By the end of the day, we were not far from where we’d started and decided to deploy a prototype hydrophone buoy that Chris Tremblay is testing in collaboration with Melissa Omand from URI. The hydrophone is set at 30 meters on a weighted cable from a float that is tracked using Iridium, SPOT GPS, and the Automatic Identification System (AIS) . We deployed the buoy and drifted most of the night not too far away. The ship was able to track the buoy easily with AIS up to six nautical miles away.

North Atlantic right whale photographed from the R/V Connecticut. Photo Credit: Amy Warren
On the morning of the 9th, we were again in calm seas and the buoy was only about four miles away. We decided to re-survey the area during the morning. With no whales sighted, we returned to pick up the buoy and continue surveying to the north where the plane reported a group of three to five whales on Tuesday. Buoy retrieval was successful and done before the seas picked up. The buoy was deployed overnight. We headed north to survey, finding another two right whales. One of them was ID’d as biopsy target, Eg#3297. Seas are too rough to launch. Interestingly, this whale was seen in Cape Cod Bay in April. One of the right whales was exactly in the shipping lanes with four ships inbound! We called the U.S. Coast Guard, Long Island sector and requested a Broadcast Notice to Mariners. They complied without question, all very good. By nightfall, we were heading into Woods Hole to run from weather and to let Chris and Genevieve troubleshoot some components of the sonobuoys. We snuck into Woods Hole around 2am, and were at the NEFSc’s Woods Hole Laboratory dock almost all day.
Some notes about the waters surveyed on May 7th – 9th: There is a LOT of fishing gear out here! With a lot of feeding whales around. The aerial surveys have been documenting whales in this area for some time now. There also seems to be a good bit of Calanus here. We’ve done five bongo tows near feeding whales, all producing good catches of Calanus. Most exciting is that Leah Crowe had identified all of our right whales and two of them are VERY interesting! One is #1145 (also known as Grand Teton), who to the best of our knowledge has not been seen since 2010 and another is #1950, who to the best of our knowledge has not been seen since 2015. We believe that neither of these whales were seen in Cape Cod Bay this spring. Both are adult females with a calving history.
On May 11th, we headed out into the Great South Channel (GSC). The NEFSC aerial team surveyed there on the 9th and found no whales. Since it was the only area with any workable weather, we decided to head out and sample for zooplankton at some of Mark Baumgartner’s historical sampling stations…back when right whales actually came into GSC in May. Our samples were interesting in that they consisted of mostly slimy sludge (science speak from someone who only looks at mega fauna), little discernable Calanus, some jellies, and a few other invertebrates. The slimy sludge was near impossible to clean off of the bongo nets. Chris and Gen deployed one sonobuoy and heard a couple of sei whale down sweeps. After dissecting weather forecasts very meticulously, Captain Marco agreed to head to George’s Bank overnight!
On May 12th, we awoke on George’s Bank to fairly good sea conditions….and rain. We knew stormy weather was coming, so were fairly judicious with our time. We began to survey, watching from the ship’s bridge. Gen and Chris deployed another sonobuoy in a location that we’d come near again on the next track line. They heard sei and humpback calls. Around 1300hrs, we got into sei whale soup! Spectacular sight with sei whales skim feeding, surfacing in every direction, oh, and there are a few humpbacks mixed in …..and wait for it….there’s a right whale! We did our best to get close to the right
whale. It was fluking about every nine minutes, so feeding deeper. We had rain and fog and sei soup. We never got close to the right whale. As we tried to leave the area and continue on our track we got into another area of sei whales, and yes, found another single right whale. Sea conditions were holding for us nicely, but we knew we had to make a dash back to Nantucket Sound, at 10 knots. Gen and Chris heard sei, humpback, and probable right whale calls on a sonobuoy deployed near the first aggregation.
Both common and white-sided dolphin were also seen and heard.
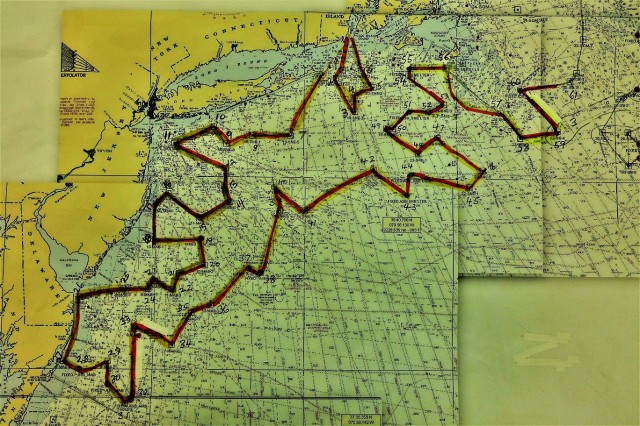
We are currently headed to Avery Point, CT, the ship’s home port, to take on fuel and hide from this weather. See map below for overview of our efforts to date.
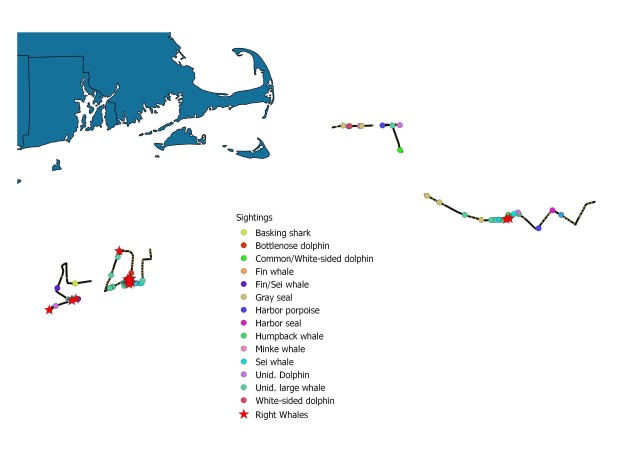
Map showing location of marine mammal sightings through May 12, 2019. Image credit: NOAA Fisheries/Elizabeth Josephson
Lisa Conger
Aboard R/V Connecticut
Spring 2019 Right Whale Cruise